Project Z01 - Computational Chemistry: Theory and Modeling of Cooperativity in Chemical Systems (Neugebauer/Mück-Lichtenfeld/Waller)
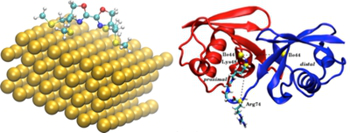
Project Z01: Partially optimzed structure (LDA/tier 1 NAO) of a Bisoxazoline Ligand on a Au(111) Surface (left), Ubiquitin-Dimer with Isopeptide Linkage (right).© SFB 858 Within the SFB 858 we describe the phenomenon "Cooperativity" on different levels of complexity of the respective systems. Systematic generation, manipulation and understanding of cooperative effects in chemical reactions afford detailed knowledge about the structural and energetic relationships on the molecular level. Modern quantum chemistry based molecular modeling methods have developed to powerful and reliable tools in recent decades for planning and interpreting chemical reactions.
Besides standard quantum chemical methods we will make use of subsystem density functional theory approaches, as developed in the Neugebauer group. QM/MM methods will be employed for larger systems. These theoretical methods provide tools for the analysis of cooperativity based on subunits or fragments of the systems under investigation.
Project Z03 - How to communicate and mediate Chemistry ? – Development and Evaluation of a Vocational Preparation Seminar (Marohn)
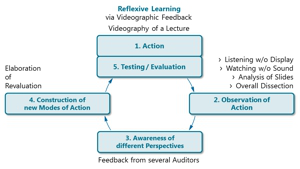
Project Z03: Reflexive Learning Circle (rf. Korthagen et. al.).© SFB 858 The aim of this project is to develop, implement and evaluate a vocational preparation seminar. The members of the IRTG will be trained and guided to communicate scientific contents considering recent results of educational research. They shall modulate methods and wording with respect to the envisaged receiver auditorium. For this purpose genuine situations will be utilized or simulated (progress reports, symposium contribution or communication with non-scientific involved persons). The conception of the seminar will be flanked by interviews with scientists and within industry in order to determine required skills and potential professional surroundings (e.g. as disciplinarian / supervisor).
Project A01 - Cooperative Reactions of „non quenching“ Lewis-Acid/-Base Pairs with unsaturated Substrates (Erker/Eckert)
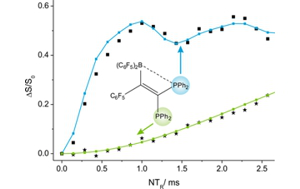
Project A01: 31P{11B} REAPDOR-Experiment at a FLP with two P-Species. Simulation delivers P···B-distances of 2.04 and 4.25 Ǻ.© SFB 858 In the first period within project A01 cooperative reactions of frustrated Lewis pairs (FLPs) with conjugated enynes and diynes were found to give cyclic allene and butatriene derivatives. Some FLPs showed coordination behavior toward isonitriles that remotely remembered that of metal complexes. Now it is envisaged to develop new FLP types with alkenyl borane functionalities that can react with alkenes and alkynes either as "normal" P/B FLPs or alternatively as vinylogous P/C FLPs. The latter would yield products via borataethene formation. The new products shall be investigated by multinuclear solid state NMR spectroscopy and shall serve as substrates for the development of new NMR pulse sequences. Furthermore, solid state NMR will be utilized as a powerful tool to give insight to heteronuclear distances, bonding modes and may therefore complete the understanding of reaction modes.
Project A02 - Compounds Consisting of Group 13 Lewis Acids and Nitrogen based Lewis Bases for Bifunctional Activation (Uhl)
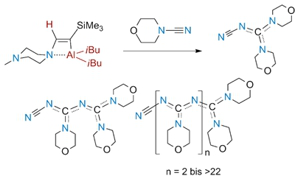
Project A02: Cyanamides give chain oligomers instead of the energetically strongly preferred 1,3,5-triazines.© SFB 858 Active Lewis pairs based on Al and N atoms were obtained by hydroalumination of ynamines or of a hydrazone. Strained AlC2N heterocycles with long Al-N bonds were isolated from the first type of reactions, while the second one yielded a monomeric hydrazide with a three-membered AlN2 ring. Cleavage of endocyclic Al-N bonds resulted in the formation of highly reactive intermediates which were applied for the cooperative activation or coordination of terminal alkynes or heterocumulenes. Cyanamides gave unique reactions in which chain oligomers instead of the energetically strongly preferred 1,3,5-triazines were formed.
Project A06 - Amphiphilic Tetrylenes in (asymmetric) Catalysis (Glorius/Hahn)
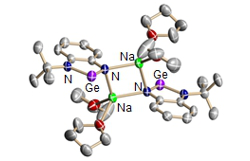
Project A06: Dimeric Germylene Anion.© SFB 858 The heavier analogues to N-heterocyclic carbenes (NHCs), the N-heterocyclic stannylenes (NHSn), germylenes (NHGe), and plumbylenes (NHPb) are the key compounds within this project. The singulett tetrylenes feature a EII-Atom with a Lewis basic sp2-orbital, e.g. to coordinate a metal center, along with a Lewis acidic p-orbital. The heavier tetrylenes are more electrophilic in comparison to their NHC pendants, which makes the empty p-orbital at the EII-element more important. This amphiphilicity may lead to the simultaneous exhibition of two different (catalytic) functionalities at the same atom side and shall be explored for cooperative catalysis or substrate activation.
Project A07 - Heteropolynuclear Carbene Complexes with cooperatively interacting Metal Centers (Hahn)
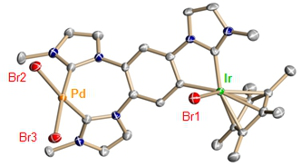
Project A07: X-Ray structure of a Heterobimetallic Complex based on the Topology of Polycarbene Ligands.© SFB 858 This project is focusing on the preparation of (hetero)bimetallic und polymetallic carbene complexes containing cooperating metal centers and their application in homogeneous catalysis. The successful studies of the first project period will be continued and will be extended by applications in the field of cooperative catalysis. Two different synthetic methods for preparation of the heterobimetallic complexes will be applied. Firstly, the defined and proper geometric positioning of NHC-donors in different frame structures allows for the formation of heterobimetallic complexes. The preference of selected metal ions for a predefined coordination geometry will play a key role for the selective complex formation. The second approach is based on the use of ligand precursors containing azole and azolium groups. The oxidative addition of the azole groups to transition metals generates carbene complexes featuring an NHC ligand with an unsubstituted ring-nitrogen atom. These reactive ligands can be protonated or alkylated while coordinated to a metal center. The azolium function can subsequently be deprotonated and metallated with different metal ions to yield selectively heterobimetallic complexes.
Project A09 - Are Stereoelectronic Effects Additive or Cooperative? (Gilmour)
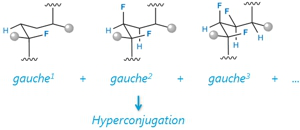
Project A09: Subsequent Analysis of the Cooperative Nature of stereoelectronic Effects.© SFB 858 Stereoelectronic effects are abundant in nature and are also key elements in organic chemistry. The goal of the project is to explore whether stereoelectronic effects are additive or cooperative. To this end, various multi-vicinal fluorinated alkanes which are constructed from CHF-entities with defined configuration will be studied. These little explored functionalities will eventually be incorporated into catalytically and biologically interesting compounds. Realization of this project demands for a multidisciplinary approach within the frame of the SFB: syntheses and structural analysis will challenge the analytical institutions of the WWU Münster and also computational chemistry will be used to understand and quantify the addressed interactions. Finally, the novel compounds will be applied to enantioselective catalysis.
Project A10 - Imidazolin-2-ylidenaminophosphines as Cooperative Ligands for the Activation of Strong Bonds (Dielmann)
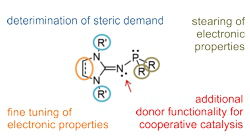
Project A10: Properties of IAP-Ligands.© SFB 858 Project A10 focusses on the cooperative activation of substrates bearing strong enthalpic sigma-bonds using metal complexes with imidazolin-2-ylidenaminophosphines (IAPs). IAPs can be considered as very well suited donor ligands with basic imino nitrogen atoms at the phosphorous atom, providing cooperative binding sites. Bifunctional activation of E-H-bonds (E = H, N, O, C) and bimetallic activation of C-E-bonds (E = N, O, F, CN) mediated by metal complexes with IAP-ligands shall be investigated.
Project B01 - Cooperative Catalysis at Surfaces – A Combinatorial Approach (Studer)

Project B01:TEM images obtained for well-dispersed PdNPs decorated with Polymers.© SFB 858 The first part of the project is dealing with the preparation of mesoporous SiO2 nanoparticles which carry orthogonally addressable functional entities at the surface, which after chemical manipulation allow for cooperative catalysis. The inorganic/organic hybrid materials are eventually used as recyclable catalysts.
The second part is focusing on the development of a new approach for the preparation of defined nanoparticles. Polymers readily obtained via nitroxide mediated radical polymerization are applied as reducing reagents to the generation of the nanoparticles from the corresponding transition metal salts. The polymer stabilized nanoparticles are eventually used as catalysts in different reactions.
Project B02 - 2D Reactions at Surfaces (Fuchs, Studer)

Project B02: Glaser Coupling at Surfaces (Cover in ACIE 2013, 52, 4024-4028.)© SFB 858 This project is focusing on the development of two-dimensional reactions at different metal surfaces („on surface chemistry“). To this end, the reaction component is adsorbed under UHV conditions to the metal surface. Heating of the monolayer leads to covalent bond formation. The Glaser-coupling developed in the first project period as a valuable on surface process will be applied to build up graphene-type networks. Along with the Glaser-coupling, azide/alkyne cycloadditions and aldehyde couplings will be used for the preparation of two-dimensional network structures. Mechanistic studies on 2D reactions using DFT calculations will complement the experimental work.
Project B03 - Chemical Reactions of Organized Lipophilic Molecules at Surfaces (Chi, Erker, Fuchs)
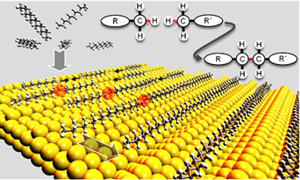
Project B03: Alkane Polymerization at a Au(110) Surface.© SFB 858 In the first period we have found that long chain linear alkanes can be polymerized on a Au(110) surface at elevated temperature with elimination of dihydrogen under UHV conditions. We now want to extend this new reaction to the polymerization of functionalized compounds containing long chain alkyl groups by twofold C-H activation on the gold surface. This would give novel ways to new variants of polyesters, polyketones, polyamides etc. Can the gold surface also be utilized for other C-C coupling reactions, e.g. for the formation of dibenzopentalene derivatives by gold catalyzed isomerization of the respective acetylenic precursors?
Project B04 - Cooperativity within the Dynamic Organization of Membrane Lipids by Peripherically Associated and Integral Proteins (Gerke, Galla, Heuer)
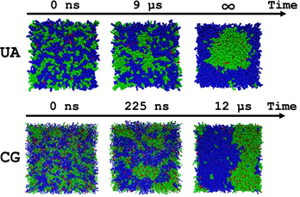
Project B04: Simulated Generation of Lipid Microdomains: United Atom (UA) vs. Coarse Grained (CG). © SFB 858 Cellular membranes show a high degree of self-organization leading to spatiotemporal generation of subdomains. This dynamic organization is of key importance for membrane transport and for transduction of biological, chemical and mechanical signals of the extracellular environment into the cell. Fundamental aspects of this membrane organization will be studied within project B04 using a combined experimental and theoretical approach. Lipid phase separation in solid supported membranes will be investigated experimentally using atomic force microscopy and phase separation in large vesicles will be investigated by using fluorescence marked lipids. The study of the formation of domains induced by the annexin protein family will be a central point of this project. For integral membrane proteins project B04 focusses on the so called ABC transporter multi drug resistance family. Theory supports the experimental work applying molecular dynamic simulations to study the phase behavior and the dynamic properties of the lipid compositions with and without associated proteins as well as with integral membrane proteins.
Project B06 - Biomimetic Molecular Recognition of Carbohydrates (Ravoo)
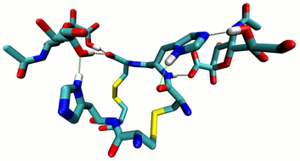
Project B06: BLYP-D3/def2-TZVP optimized Structure of a parallel Dimer Complex of HisHis with two Molecules of NANA. © SFB 858 Molecular recognition and specific binding of carbohydrates with proteins (lectins) are key steps in various physiological processes. Proteins interact with carbohydrates primarily via multiple H-bonding. The aim of the project is to prepare synthetic lectins by using a dynamic combinatorial approach. In the first project period this route was successfully used for the synthesis of a N-acetylneuraminic acid receptor, which incorporated into monolayers and also in competitive binding with natural lectins showed activity. The goal of the second period is to identify even better synthetic lectins and to thoroughly characterize the binding of them with various carbohydrates. New binding elements such as boronic acids will be included into the lectin design. Moreover, receptors should be anchored to membrane surfaces and the peptide carbohydrate interaction in these systems will be investigated.
Project B08 - DNA as Chiral Element of Organization for Catalysts in Aqueous Media (Hennecke)
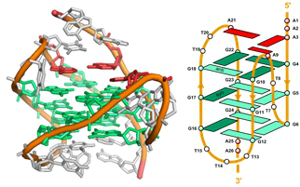
Project B08: Structure of HTel G-Quadruplex in KCl-Solution. © SFB 858 In this project we want to use DNA as a functional polymer to organize catalysts and reactants in chemical reactions. The catalytically active species that will be investigated will include mononuclear metal complexes, metal porphyrines as well as DNA-bound nanoclusters of noble metals. Such nanoclusters consisting of, for example, silver atoms can be easily prepared directly at DNA strands, but have only been used as highly fluorescent quantum dots and not as active catalysts. We plan to investigate the catalytic properties of such metal-modified DNA hybrid catalysts in aqueous solution and apply them in organic chemistry. Furthermore, we want to exploit the functional groups of DNA to orient and, if possible, activate the reactants in the catalytical reactions to allow the transfer of the chiralty of the DNA onto the reactants and enable cooperative, stereoselective reactions.
Project B09 - Influence of neighboring metal-mediated base pairs in DNA double helices on charge transfer through DNA and other cooperative effects in metal-modified DNA (Müller)
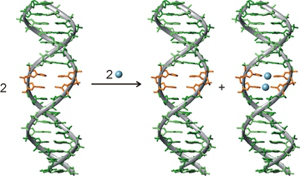
Project B09: Selective Cooperative Binding of Metal Ions in Artificial DNA Double Helices.© SFB 858 Cooperative effects in the context of the formation of metal-modified DNA will be investigated. The main focus is related to the question as to what extent charge transfer through DNA is influenced by the presence of metal-mediated base pairs. In addition, the cooperativity of the formation of neighboring metal-mediated base pairs will be studied in detail. A third aspect will be the cooperative formation of structurally defined DNA aggregates from metal-mediated DNA three-way junctions.
Project B11 - Cooperativity in Inorganic-Organic Hybrid Systems: Steering of Magnetic Properties by Orientation of Radicals (Eckert, Studer)
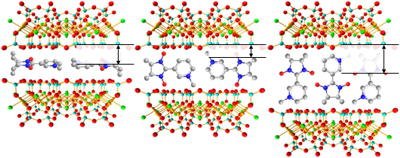
Project B11: Incorporation of Nitronyl Nitroxides in Clay.© SFB 858 The ultimate goal of the project is the synthesis of magnets based on organic radicals. Rigid conjugated polyspin systems which can be analyzed by theoretical methods will be prepared. The defined immobilization of organic radicals in different host systems should result in hybrid materials which show ferromagnetic spin spin interactions. Organic radicals will be applied as amphiphiles to construct micellar systems which via template synthesis can be used for preparation of inorganic/organic nanocomposites carrying well-ordered spin systems. Also polymer brushes containing a high spin density will be prepared. Magnetic susceptibility of all polyspin systems will be measured. Moreover, solid state NMR and EPR methods will be developed and applied to characterize and quantify spin spin interactions in these novel materials.
Project B13 - Molecular Basis for the Cooperative Action of a Helicase and a Topoisomerase Domain in Reverse Gyrase in Positive DNA Supercoiling (Klostermeier)
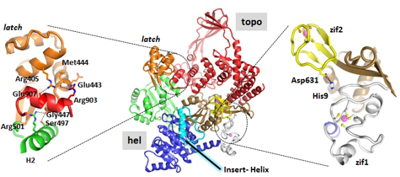
Project B13: Structure of T. maritima reverse gyrase.© SFB 858 Helicases and topoisomerases are important for the maintenance of genome stability. Reverse gyrase consists of a helicase domain and a topoisomerase domain that introduce positive supercoils into DNA in a cooperative manner. We will (1) identify key residues for the functional interaction between these domains, (2) define how individual interactions of several structural elements with DNA are coordinated in time and space, and (3) investigate the temporal coordination of cooperative conformational changes during positive supercoiling of DNA. Thereby, we will elucidate the molecular basis for the functional cooperation of these domains in reverse gyrase.
Project B14 - New Protein Chemical Tools for the Analysis of Cooperative Effects within Protein Interactions mediated by Ubiquitin-like Modifiers (Mootz)
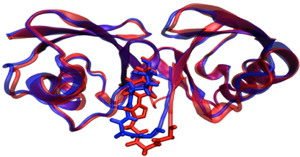
Project B14: QM/MM calculation of Ubiquitin Dimer: Native (K48 linked, red) and with synthetic Linkage (Triazole, blue). © SFB 858 The aim of the project is to investigate the role of posttranslational modifications of the ubiquitin type (ubiquitin and SUMO) in protein-protein interactions. To this end, new methods will be developed to chemically conjugate ubiquitin and SUMO to target proteins and to endow them with crosslinking moieties and fluorophores. These modifications will enable new protein biochemical studies on the specific recognition by protein binding partners and on the structure and function of chains of these posttranslational modifiers.
Project B15 - Innovative Ligands at Nanoparticles and at Surfaces (Glorius/Ravoo)
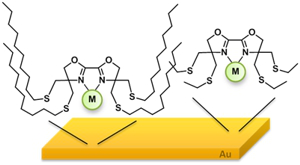
Project B15: Illustration of a lipophilzed bidentante metal complex binding to a Au-Surface.© SFB 858 Innovative carbene and thioether ligands are developed within this collaboration that bind to nanoparticles and surfaces. An efficient ligand exchange protocol allows the generation of novel carbene functionalized nanoparticles, that shall be tested in e.g. asymmetric catalysis. The nanoparticle synthesis does not involve the stabilization of the particles by N-heterocyclic carbenes, being one of the advantage of this new method and allowing the synthesis of a variety of functionalized nanoparticles and leading to more stable particles. These libraries shall be synthesized, characterized and applied in catalytic test reactions. Furthermore we have shown, that specific thioether ligands with bioxazoline moiety generate stable monolayers at gold surfaces. It has to be shown, how this special arrangement of the metal ion at the metal surface influences the hybrid’s catalytic properties. Finally, the option of utilizing our novel ligands for the development of metal nanoparticle based sensors shall be evaluated.
Project B17 - Cooperative supramolecular Homo- and Heteropolymerization of BODIPY dyes: Influence of various unconventional non-covalent forces (Fernandez-Huertas)
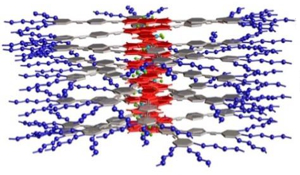
Project B17: H-Aggregation of Amphiphilic OPE-BODIPY-Derivatives.© SFB 858 BODIPY dyes have become relevant in the past two decades due to their excellent optical and photophysical properties, which has allowed their application in fields such as optoelectronics and biomedicine. In contrast, and despite their strong tendency to stack in solution and the solid state, their supramolecular aspects remain thus far limited. This proposal aims at controlling the supramolecular (co)polymerization of BODIPY dyes by exploiting various unconventional non-covalent forces in a cooperative fashion. These include: 1) weak CH hydrogen bonding or metallophilic interactions and 2) arene-perfluoroarene or CH··CF interactions.
Project aB18 - Chemo-enzymatic manipulation of RNA: Harnessing cooperative effects to achieve sequence-specificity (Rentmeister)
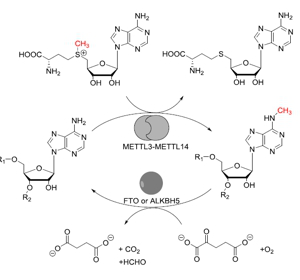
Project aB18: Reversible methylation of adenosine in mRNA of higher eukaryotes.© SFB 858 Sequence-specific manipulation of RNA is of particular importance for many molecular biology applications but also to study regulation of gene expression at the post-transcriptional level. The recent discovery of reversible methylation of adenosine in messenger RNA of higher eukaryotes represents a potential new regulatory mechanism at the post-transcriptional level. To date, it is straightforward to up- or downregulate the general mRNA methylation level by overexpressing or knocking down expression of genes involved in the methylation or demethylation reactions. The precise manipulation of methylation of one specific nucleotide however is currently not possible, impeding assignment of the role of single methylations.
In this project, we will create fusion constructs of RNA-modifying enzymes and RNA-binding proteins or RNA-protein complexes with tunable sequence-specificity. We will focus on enzymes involved in demethylation of m6A and test different RNA-sequence recognition approaches to achieve site-specific manipulation of specific RNAs.

